GPS2 Deficiency Triggers Maladaptive White Adipose Tissue Expansion in Obesity via HIF1A Activation
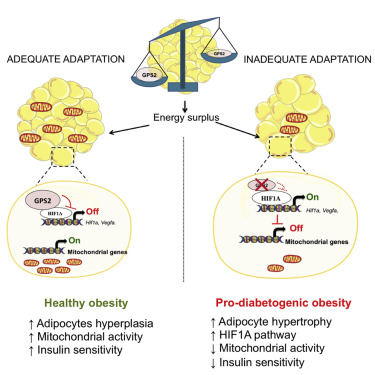
Hypertrophic white adipose tissue (WAT) represents a maladaptive mechanism linked to the risk for developing type 2 diabetes in humans. However, the molecular events that predispose WAT to hypertrophy are poorly defined. In this paper, Drareni et al. demonstrate that adipocyte hypertrophy is triggered by loss of the corepressor GPS2 during obesity. Adipocyte-specific GPS2 deficiency in mice (GPS2 AKO) causes adipocyte hypertrophy, inflammation, and mitochondrial dysfunction during surplus energy. This phenotype is driven by HIF1A activation that orchestrates inadequate WAT remodeling and disrupts mitochondrial activity, which can be reversed by pharmacological or genetic HIF1A inhibition. Correlation analysis of gene expression in human adipose tissue reveals a negative relationship between GPS2 and HIF1A, adipocyte hypertrophy, and insulin resistance. The authors propose therefore that the obesity-associated loss of GPS2 in adipocytes predisposes for a maladaptive WAT expansion and a pro-diabetic status in mice and humans.
To read the full paper, head to the following link:
https://www.sciencedirect.com/science/article/pii/S2211124718313032?via%3Dihub





